The Anthrocell Expression System: ACX™
The Anthrocell Expression System (ACX™) is a 4th generation expression platform for production of biopharmaceutical protein drugs.
Derived from mature human lymphocyte cells, ACX™ is 100% human. Biopharmaceutical proteins produced with ACX™ are structurally identical to ones that are produced by the human immune system, and thus able to engage effectively with that immune system to achieve optimal treatment results.
ACX™ expressed proteins have fully human glycosylation patterns and other post translational modifications. This means that drugs produced with ACX™ have higher bio-efficacy and low immunogenicity. Because they can achieve the desired biological results at lower dosages, they also have less adverse side effects.
ACX™ is optimized for production of blood proteins such as monoclonal antibodies. The first 100% human protein expression system for commercial scale production of biopharmaceutical drugs, it is the best option for any monoclonal antibody project.

Performance Parameters
Stable, with high transfection rate, ACX™ can be used in the initial development phase, while with rapid growth and high expression it offers attractive economics for final production. By saving the time and effort needed to migrate between the R&D host and the final production host, ACX™ can shorten time to clinical trials and market by 6-9 months.
The ACX™ platform is viable in suspension and has been shown free of opportunistic viruses by NGS testing.
Key performance parameters include:
- Transfection rate: > 90%
- Yield at 48 Hrs: 20 μg/mL/106
- Time to Stable Clone: <3 months
- Peak Density: 35-43 x 106 cells/mL
- Doubling time: 22 to 34 Hours
- Cell Productivity: >20 picograms/cell/day

Interested in partnering with Anthrocell?
Want to know more about our technology?
Post Translational Modifications
Over the past decades, a growing body of evidence has demonstrated that the therapeutic performance of most protein-based biopharmaceuticals is significantly affected by the pattern of Post Translational Modifications (PTMs) they acquire in later stages of production.
PTMs involve the addition of various chemical structures to the protein’s core amino acid chain, which impact the protein’s final structure and thus its chemical activity. Some of the more common PTMs can be seen below:
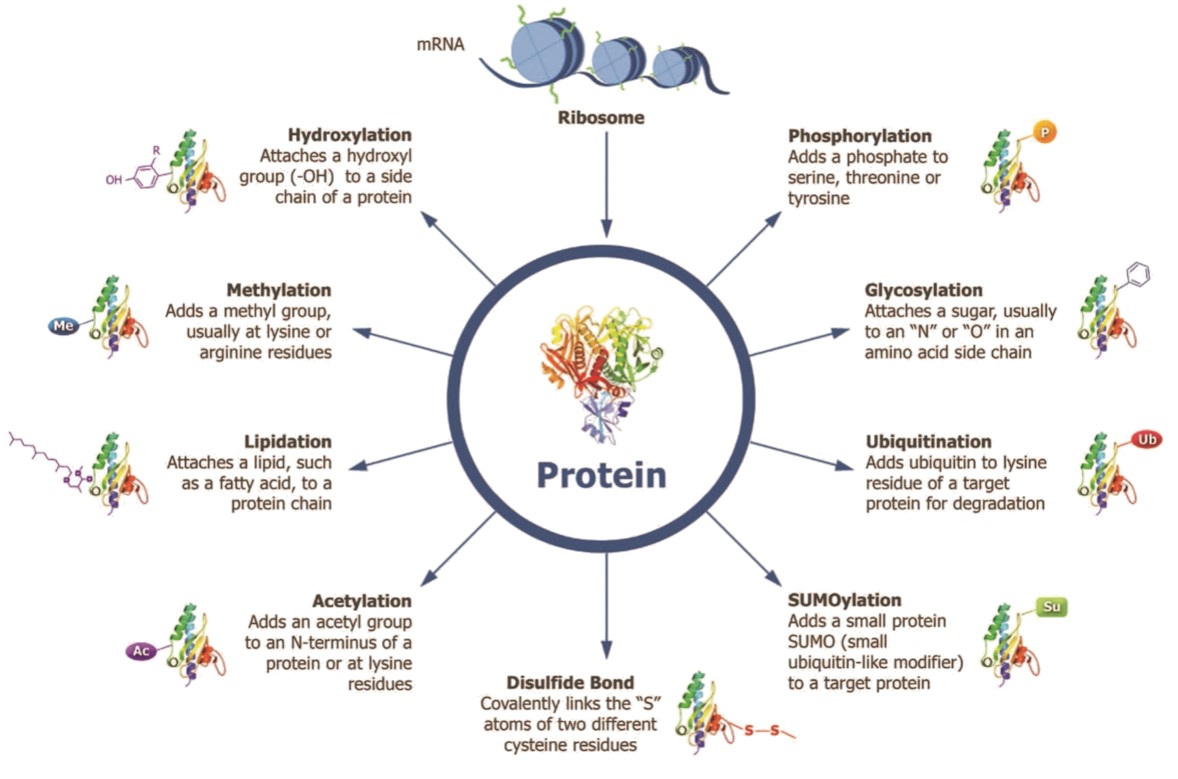
The primary determinant of what particular PTMs will be applied to a protein during expression is the internal environment of the cell in which that protein is synthesized.
Cells of different species, and cells from different tissues within a particular species, have distinct cellular environments, and will potentially result in a distinct PTM pattern. This can impact the final (tertiary) structure of the expressed protein and consequently its biologic function.
The impact of the various PTMs on the protein’s final structure can impact key aspects of how the drug affects the patient’s biological and disease processes.
In particular, PTMs impact the drug’s:
- bio-efficacy (how well the protein binds to the target)
- half-life (how long the protein lasts in the blood)
- side effects (including inflammation)
- rejection (whether the body identifies the drug as foreign and develops antibodies to it)
You can learn more about PTMs at :
Glycosylation
One of the most common Post Translational Modifications is glycosylation, the addition of various glycan residues. Because different species have very different glycosylation patterns, glycosylation is particularly sensitive to the host used for expression.
In particular, proper glycosylation has been shown to improve:
- Inflammation response
- The drug’s ability to harness immune system mechanisms such as ADCC
The CHO (Chinese Hamster Ovary) cells most commonly used for commercial production of biopharmaceuticals are known to have non-human glycosylation patterns and other PTMs.

